Although already discovered in 1992 as a form of programmed cell death (PCD) caused by pathogen infection of macrophages1, the term pyroptosis was only introduced in 2001 as a combination of the Greek words pyro (fire or fever) and to-sis (falling)2 to describe the rapid release of inflammatory cytokines such as interleukin-1β (IL-1β) and IL-18 from dying cells. Pyroptosis helps to combat intracellular infections by eliminating the affected cell and exposing the pathogen but is not limited to host defense. Some viruses, such as SARS-CoV-2, can induce pyroptosis, which contributes to the development of an excessive immune response known as the "cytokine storm".3 Over the years, several mediators of pyroptosis were identified. While initial studies showed a dependence on caspase-14, it is now clear that other caspases, such as caspases 4, 5, and 11, can also mediate pyroptosis.5
Based on the activating caspases, the signaling pathways can be divided into canonical (caspase-1) and non-canonical signaling pathways (caspase-4, 5, and 11).6 In the canonical pathway, caspase-1 is activated by inflammasomes such as the NLRP3 inflammasome, which are multimeric protein complexes reacting to different stimuli such as damage- or pathogen-associated molecular patterns (DAMPs or PAMPs respectively). In the non-canonical pathway, the pyroptosis-triggering caspases directly serve as receptors for intracellular lipopolysaccharide (LPS) from Gram-negative bacteria, activating the NLRP3 inflammasome in a secondary step.7
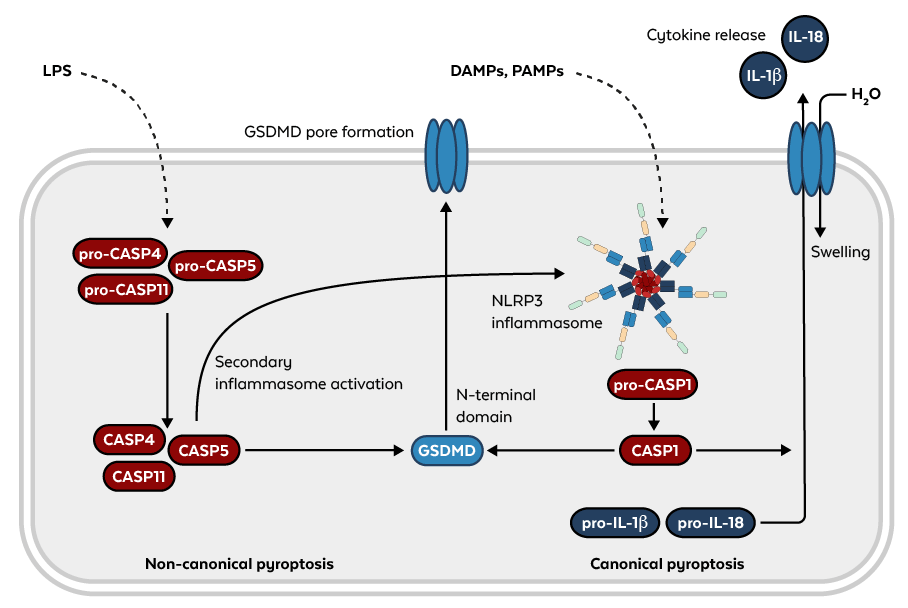
Figure: Non-canonical (left) and canonical (right) pyroptosis pathways.
The effector of pyroptosis that ultimately leads to cell death by membrane rupture was not identified until 2015. Gasdermin D (GSDMD), as it is called, belongs to a protein family with a conserved structure. GSDMD is cleaved and activated by caspases, releasing its N-terminal gasdermin domain and forming a pore in the plasma membrane. Caspase-1 cleaves pro-IL-1β and pro-IL-18, generating mature cytokines that are then released through these pores prior to H20 influx and membrane rupture.8 This rupture might be the result of another effector of pyroptosis that has been identified recently. Ninjurin-1 (NINJ1) is a cell surface protein that facilitates the rupture of the plasma membrane in a cookie-cutter-like mechanism.9 Since we are only beginning to understand the various components and interactions of this pathway, the future will hold many more insights.
Rockland antibodies are rigorously tested and validated for specificity and sensitivity to ensure accurate and reliable results. With our extensive collection of antibodies targeting key components of the pyroptosis pathway, including Caspase-1, IL-1β, and NLRP3, you can trust Rockland to provide the tools you need to advance your research.
Pyroptosis Antibodies
| Product | Clonality | Reactivity | Application |
| ASC Antibody | Polyclonal | Human | WB, IHC, ELISA |
| Caspase-1 Antibody | Polyclonal | Human | WB, IHC, ELISA |
| Caspase-1 Antibody | Polyclonal | Human | WB, IF, IHC, ELISA |
| Caspase-1 Antibody | Monoclonal | Human, Mouse | WB, IF, IHC |
| Caspase-4 Antibody | Polyclonal | Human, Mouse | WB, IF, IHC, ELISA |
| Caspase-4 Antibody | Polyclonal | Human | WB, IF, IHC, ELISA |
| Caspase-5 Antibody | Polyclonal | Human | WB, ELISA |
| Caspase-5 Antibody | Polyclonal | Human | WB, IF, IHC, ELISA |
| IL-1 Beta Antibody | Polyclonal | Mouse | WB, IF, IHC |
| IL-1 Beta Antibody | Polyclonal | Human | WB, IHC, ELISA Functional Assay |
| IL-1 Beta Antibody | Polyclonal | Human, Dog, Primate | WB |
| Mouse IL-18 Antibody | Polyclonal | Mouse | WB, IF, IHC |
| NALP3 Antibody | Polyclonal | Human, Mouse | WB, IF, IHC, ELISA |
| NINJ1 Antibody | Polyclonal | Human, Mouse, Rat | WB, IHC, IF, ELISA |
| NLRP3 Antibody | Polyclonal | Human, Mouse, Rat | WB, IF, IHC, FC |
Pyroptosis Assays
| Product | Reactivity | Detection Range |
| Human IL-1 beta ELISA Kit | Human | 3.9 pg/ml - 250 pg/ml |
| Mouse IL-1 beta ELISA Kit | Mouse | 12.5 pg/ml - 800 pg/ml |
| Rat IL-1 beta ELISA Kit | Rat | 31.2 pg/ml - 2000 pg/ml |
| Human IL-18 ELISA Kit | Human | 31.2 pg/ml - 2,000 pg/ml |
| Rat IL-18 ELISA Kit | Rat | 15.6 pg/ml - 1000 pg/ml |