TrueBlot
Immunoprecipitation (IP) and Western blot (WB) protocols provide highly specific results but often suffer from poor or non-specific binding, high background, and heavy/light chain interference. Rockland’s TrueBlot® line of products consists of both TrueBlot® IP beads and TrueBlot® monoclonal secondary antibodies, which combat these common experimental issues by providing increased specificity, low background, and enhanced accuracy to provide publication-quality IP Western blots without interference from contaminating IgG.

How TrueBlot® Secondaries Work
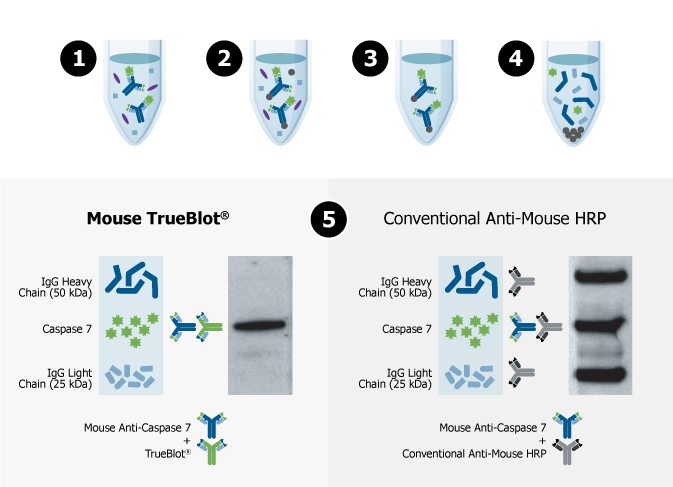
When proteins are isolated using IP, a capture antibody binds to the target protein (Step 1), Protein A- or Protein G-coated magnetic beads are added to immobilize the antibody-target protein complex (Step 2) and the undesired material is washed away (Step 3). The final steps in a conventional IP typically include denaturation of the proteins using heat and a reducing/denaturing agent (Step 4).
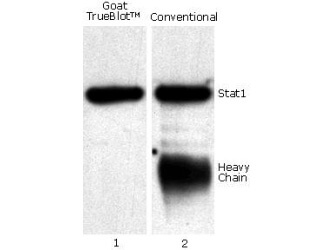
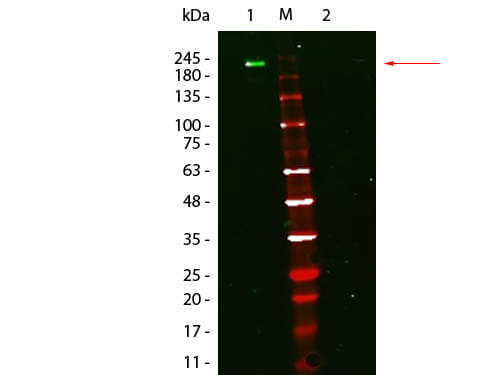
When the eluted IP sample is separated via SDS-PAGE (Step 5), probed with the same primary antibody used in IP, and detected with conventional secondary antibodies in Western blot, both the heavy and light chain of the IP capture antibody can be detected (Step 5; right panel), which can obscure your protein of interest, specifically if it is similar in size to the heavy or light chain.
TrueBlot® secondary antibodies only recognize primary antibodies in their native (non-reduced) state, eliminating interference from heavy and light chain bands (Step 6; left panel) to provide accurate, publication-quality Western blots of immunoprecipitated samples.
Featured TrueBlot Products
Selecting TrueBlot® Products
Rockland’s TrueBlot® product line allows researchers to generate reproducible, publication-quality IP-Western blots easily. But with so many products to choose from, it may be hard to determine which TrueBlot product is best for your Western blot assay. Use our handy flowchart to navigate to the best TrueBlot product based on your specific assay, detection method, and more.
DOWNLOAD FLOWCHART