2D Western Blot
2D Western Blot
Rockland offers complete 2D Western blotting services in chemiluminescent or fluorescent format. 2D Western blotting is the standard procedure for determining HCP coverage for biopharmaceutical product approval. It combines total protein detection with the use of specific HCP antibodies in high-resolution imaging. 2D Western blots can also be used to visualize post-translational protein modifications such as phosphorylation using anti-phospho antibodies. Whether you need support in a complex HCP assay project or are only outsourcing 2D Western blots, you can be confident when you partner with Rockland.
HCP Coverage Analysis Using 2D Western Blot
A crucial prerequisite in the drug manufacturing process is an efficient analysis of Host Cell Protein (HCP) impurities that result from process-specific expression conditions as well as downstream purification procedures. Present guidelines call for minimum levels of HCP contaminants left behind during the purification process. To evaluate the presence of residual HCP contamination of the final biopharmaceutical product, polyclonal antibody development against null HCP material provides a valuable tool for detecting product impurities. Coverage assessment of the polyclonal antibody can be evaluated using 2D Western blots as outlined below.

2D Gel Electrophoresis
In the first step of a 2D Western blot, proteins are separated in two dimensions according to their isoelectric point (isoelectric focusing, IEF) and molecular weight (SDS-PAGE). This gel can be stained to visualize total protein and be available for differential analysis. It also allows individual spots to be cut directly from the gel for mass spectrometry studies.
Fig. In-gel protein stain of a 2D gel with CHO-HCP sample
2D Western Blot
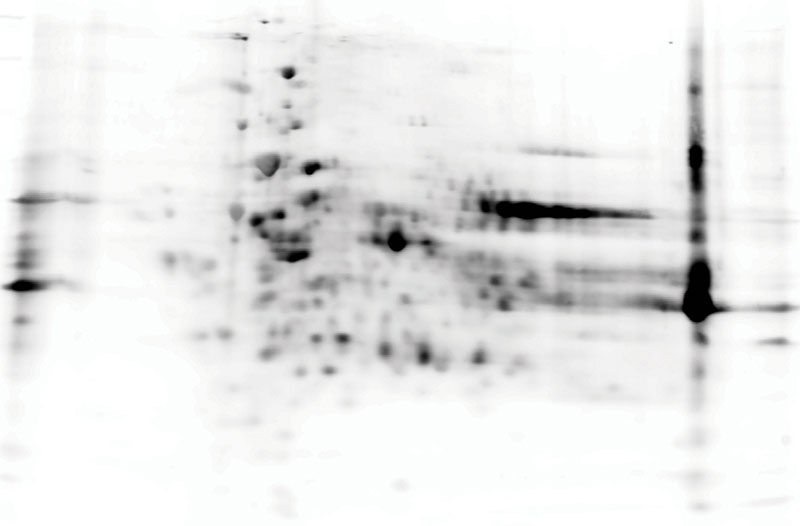
After 2D gel electrophoresis, proteins are transferred to a PVDF membrane and stained with a combination of an anti-HCP antibody and a secondary antibody coupled to HRP or a fluorescent dye.
Fig. Immunostaining of a Western blot using an anti-CHO HCP antibody
Conventional Coverage Analysis
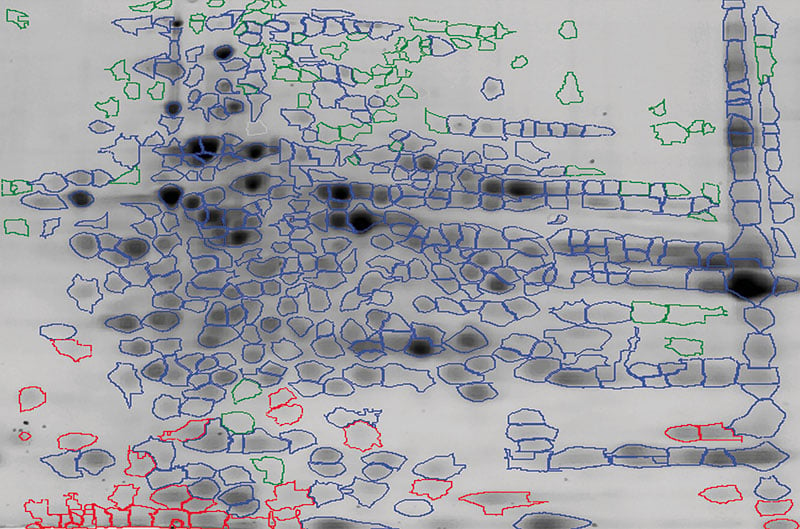
In a conventional coverage analysis, total protein from the in-gel stain is overlayed with the immunostained Western blot. The evaluation of the antibody coverage uses a combination of automated software and the expertise of our scientists.
Fig. HCP spot map using an overlay of the 2D in-gel stain and the 2D Western blot image
2D Differential In Blot Electrophoresis (2D-DIBE)
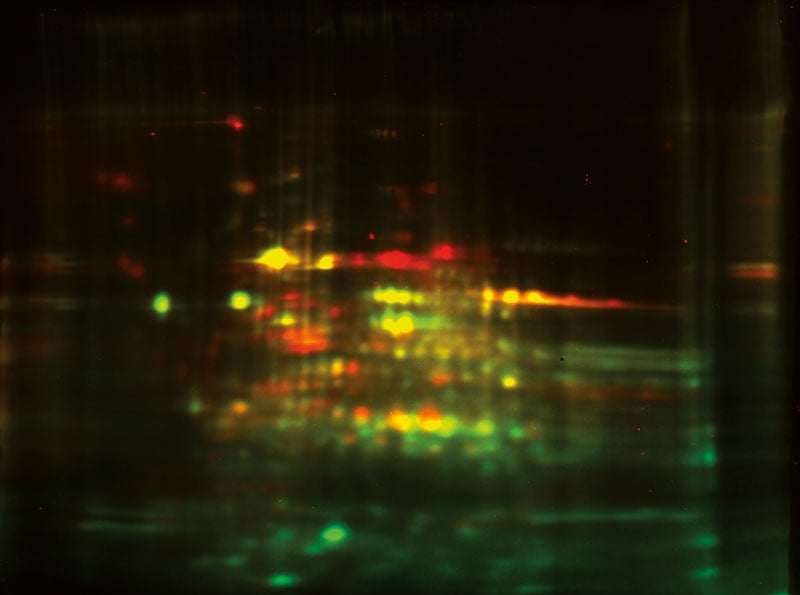
2D-DIBE utilizes a fluorescent multiplexing approach where the proteins and antibodies are tagged with different cyanine dyes. Total protein and antibody coverage can be analyzed on the same membrane. This method thereby bypasses the need for an alignment step (Gel to WB) leading to higher accuracy. Contact us if you want to learn more about 2D-DIBE.
Fig. In-blot total protein (Cy3™-labeled: green) merged to anti-CHO HCP detection (Cy5™-labeled: red). The yellow color indicates coverage.
Method Comparison
| ELISA | 2D Western blot | 2D-DIBE | |
| HCP detection | ✓ | ✓ | ✓ |
| HCP coverage evaluation | - | ✓ | ✓ |
| Rapid turnaround | ✓ | - | - |
| Gel staining | - | Coomassie, fluorescent, silver | Not needed |
| Immunodetection method | Chromogenic, fluorescent, chemiluminescent | Chromogenic, fluorescent, chemiluminescent | Fluorescent |
| PTM detection | - | ✓ | ✓ |
| Total protein and target detection on the same membrane | - | - | ✓ |
| WB and Gel alignment needed | - | ✓ | - |
| Accuracy | +++ | ++ | +++ |

Melanie™ DIGE & Coverage
For high-quality 2D Western blot coverage assessments, Rockland utilizes the Melanie™ DIGE and Melanie™ Coverage software from the SIB Swiss Institute of Bioinformatics. These analysis tools combine accuracy, productivity, reproducibility, data-driven insights, and regulatory compliance to ensure quick and efficient quantification of similarities between antigen standards and coverage of antibodies to those antigens.
Trust Rockland and the Melanie software from SIB to bring your 2D gel and Western blot analyses to the next level.
Exceptional
Accuracy
Melanie ensures exceptional accuracy when comparing spots between images, guaranteeing truly meaningful and dependable coverage results. With precise spot comparisons, you can trust the reliability of the data analysis.
Reproducible Coverage Analysis
Greater reproducibility is achieved in coverage analysis with consistent and reliable results across multiple analyses. By minimizing user bias, our team is confident about making informed and objective decisions to keep assay development on track.
Data-driven
Insights
Melanie enables data-driven insights, empowering in-depth analysis and presenting clear visual representations of the results.
Regulatory Submission Readiness
With Melanie, comprehensive PDF exports are ready for regulatory submissions to health authorities like the FDA and EMA. Benefit from flexible data export options that allow you to build a strong dossier, including all essential information required for compliance.
Interested in 2D Western Blot Services?
We can help move your program forward—contact us to find out how.