What Are Endotoxins?
As early as the end of the 19th century, scientists such as Albert Charrin and Armand Ruffer proved with simple but effective experiments that filtered cultures of bacteria can induce fever in the absence of whole cells.1 The presence of endotoxins was later discovered by bacteriologist Richard Friedrich Johannes Pfeiffer, who found that parts of the bacteria themselves, in his case Vibrio cholerae, can cause intoxication.2
We know today that this effect is mediated by lipopolysaccharide (LPS), which is an important component of the outer membrane (OM) of Gram-negative bacteria (Figure 1). This means that, contrary to what the name suggests, endotoxin is found on the outside of bacteria.
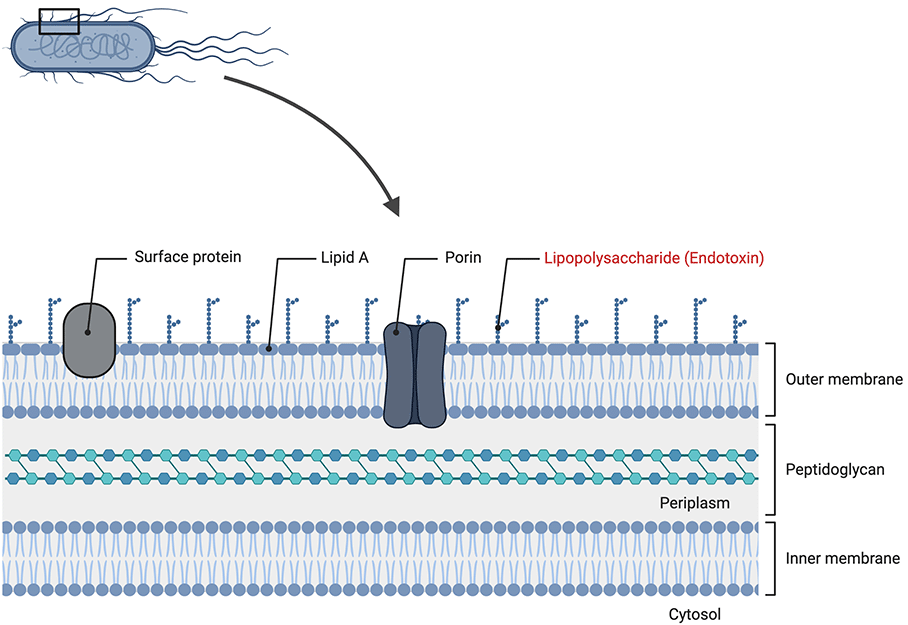
Figure 1: Endotoxin, also known as lipopolysaccharide (LPS), is a major component of the outer membrane of Gram-negative bacteria that is released upon cell lysis or as part of outer membrane vesicles (OMV) (Created with BioRender.com).
LPS provides the essential permeability barrier and covers, for example, up to 75% of the surface of E.coli. This makes it the most abundant antigen on the cell surface. LPS is indispensable for the viability of most Gram-negative bateria.3 In mammals, LPS can cause a pyrogenic (Fever response) reaction and affects many cellular functions through the induction of inflammatory cytokines (Reviewed in Cavaillon 2017).
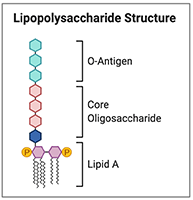
The classic lipopolysaccharide structure, shown in the figure on the left, consists of an O-side chain (O-antigen) that greatly varies between species, an intermediate region known as a core oligosaccharide, and a glycolipid (Lipid A) anchored to the outer membrane.5 The presence or absence of the O-antigen determines if the LPS is considered rough or smooth. (Figure created with BioRender.com)
What Is Endotoxin Testing Used For?
Endotoxin testing can be useful wherever there is a need to evaluate the integrity of biological and environmental samples used in research.
Examples include:
1
2
3
4
Why You Should Test Your Water Source
Ultra high purity water systems require constant and routine preventative maintenance to function properly. In the past, WFI, (water for injection) for pharmaceutical use was typically produced via distillation. This method can produce sterile and endotoxin free water. These systems are expensive and require significant capital investment and skilled technicians to maintain properly. More recently, RODI (reverse osmosis and de-ionization) have been engineered and designed that can produce high-quality water for routine laboratory use at a lower operating cost. These systems have become very popular in academic and smaller labs where the high operating costs of a WFI system are prohibitive.
There are several key points to be aware of in any high-purity water system. Endpoint/terminal sterilizing filters, typically 0.22 micron, are often used to ensure sterile water. Sterile filters by definition do not produce endotoxin-free water. In fact, if the sterile filters are not routinely replaced or regenerated via appropriate cleaning and sterilizing protocols, a biofilm of bacteria can form on the surface of these filters. So while intact bacteria are retained and technically the water is "sterile" the bacteria can be shedding copious volumes of endotoxin from any Gram-negative organisms that are established in this biofilm. These contaminated systems can, unfortunately, produce very high levels of endotoxin-contaminated water.
One should also be aware that autoclaving has no effect on endotoxin. Only dry heat can depyrogenate and destroy LPS. Also, if you autoclave a solution open to vapor exchange in a non-pure steam autoclave you run the risk of adding endotoxin to the solution being autoclaved.
If this contaminated water is then incorporated into buffers, used to formulate tissue culture supplements, or is used for any routine laboratory procedures a variety of unknown artifacts can be generated which may lead to spurious data. For example, in mammalian cell culture, TLR4 is one of several known cell surface receptors that bind endotoxin. Endotoxin-activated cells can induce and stimulate downstream cytokine production which may complicate the interpretation of any monitored cellular response.
Recommendations:
1
2
3
Suitable Sample Types
EndoAlert™ Endotoxin Plate Kits
Rockland offers two endotoxin testing kits for research use only (RUO). Both kits utilize Limulus amebocyte lysate (LAL), an aqueous extract of blood cells from the horseshoe crab (Limulus polyphemus) that reacts with LPS.
| Product | Chromgenic Lysate | Endotoxin Standard | LAL Reagent Water | Endotoxin Specific Constitution Buffer | 96-Well Microtiter Plate |
| EndoAlert™ Endotoxin Plate Kit | ✓ | ✓ | ✓ | - | ✓ |
| EndoAlert™ ES Endotoxin Plate Kit | ✓ | ✓ | ✓ | ✓ | ✓ |
Both kits are based on a chromogenic LAL reagent that is measured at 405 nm and is more sensitive and quantitative than gel clot. The EndoAlert™ Endotoxin Plate Kit is reconstituted with LAL Reagent Water. It reacts with both LPS (endotoxin) and B-D-glucan (fungal contaminants).
The EndoAlert™ ES Endotoxin Plate Kit is reconstituted with Endotoxin Specific Constitution Buffer which inactivates and blocks the Factor G pathway (fungal). The Factor C (LPS binding protein) pathway is left intact making this an endotoxin-specific assay.
Why EndoAlert™ Kits?
- Lower Cost per Assay - Designed by Trident Biosystems, which has over 70 years of experience making LAL that allows for a lean-and-mean approach to development resulting in a lower cost per assay.
- Easy-to-Use - A user-friendly protocol designed for research and clinical use eliminates the need to use complicated, USP-regulated test that are designated specifically for Pharma.
- Sensitive - Equally sensitive to any Pharma-certified kits on the market and more sensitive, stable, and functionally superior than any research-use kits on the market.
- US-Sourced - Every component of the kits is US-sourced and manufactured for a secure supply chain.
- Environmentally Sound - Raw material is collected with a high degree of care and is monitored by the Department of Natural Resources to ensure state and federal compliance.
References
- Charin, A. and Ruffer, A. (1889) Mécanisme de la fièvre dans la maladie pyocyanique. C. R. Hebdomadaires Soc. Biol., 9, 63–64.
- Pfeiffer, R. (1892). Untersuchungen über das Choleragift. Zeitschrift für Hygiene und Infektionskrankheiten, 11, 393-412.
- Klein, G., & Raina, S. (2019). Regulated Assembly of LPS, Its Structural Alterations and Cellular Response to LPS Defects. International journal of molecular sciences, 20 (2), 356.
- Cavaillon J. M. (2018). Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon : official journal of the International Society on Toxinology, 149, 45–53.
- Avila-Calderón, E. D., Ruiz-Palma, M., Aguilera-Arreola, M. G., Velázquez-Guadarrama, N., Ruiz, E. A., Gomez-Lunar, Z., Witonsky, S., & Contreras-Rodríguez, A. (2021). Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Frontiers in microbiology, 12, 557902.