EndoAlert™ Endotoxin ES Plate Kit
KMA-0200
1 Kit
ELISA
This product is discontinued
Product Details
EndoAlert™ ES Endotoxin Plate Kit - KMA-0200
Endotoxin Test Kit, Endotoxin level, Endotoxin Detection, bacterial Endotoxin, Limulus Amebocyte Lysate (LAL)
ELISA Kit
Target Details
This kit contains: 96-well plate, endotoxin standard, chromogenic lysate (~60 reactions), LAL reagent water, endotoxin specific buffer. See kit protocol for complete details. The EndoAlert™ ES Endotoxin Plate Kit is specifically formulated to detect and react only with endotoxin and is unreactive to (1,3) B-D glucans which are cell wall components of fungi. The EndoAlert™ ES Endotoxin Plate Kit provides a level of Endotoxin only in relation to the standard. It is NOT specific to the species of gram-negative bacteria which is the source of the Endotoxin in the sample. To increase accuracy of the test when the source of Endotoxin is known, use a purified Endotoxin from that species.
Application Details
ELISA
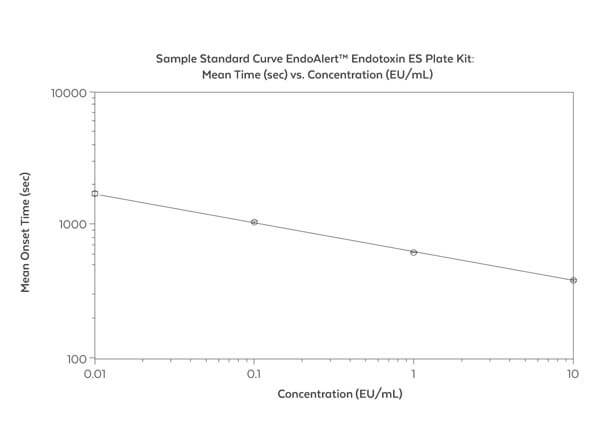
Endotoxin level detection. See kit protocol for complete details. The detection ranges from 0.01 - 10 Endotoxin units (EU/mL). Incubation 60 mins.
Formulation
1 Kit
Shipping & Handling
Wet Ice
Store all kit reagents at 2-8 °C in the dark. Avoid storing kits for extended periods (more than 24 hours) at room temperature. See kit protocol for complete details.
See kit insert for complete instructions.
The EndoAlert™ ES Endotoxin Plate Kit is a kinetic, colorimetric assay for the quantitative determination of bacterial Endotoxin in aqueous solutions. Endotoxin, a bacterial lipopolysaccharide, is one of the major cell wall components of most gram-negative bacteria. The Endotoxin ES Plate Kit detects low levels of Endotoxin and is therefore a useful tool to assess the integrity of biological and environmental samples. The detection ranges from 0.01 - 10 Endotoxin units (EU/mL). The EndoAlert™ ES Endotoxin Plate Kit is a quantitative version of the reaction first described by Levin and Bang in 1968. The test is based upon an enzymatic cascade where Endotoxin activates Factor C in Limulus Amebocyte Lysate (LAL) which in turn activates Factor B. Factor B activates Proclotting Enzyme which then activates Clotting Enzyme. A colorless synthetic peptide substrate is hydrolyzed by Clotting Enzyme to generate a yellow color which can be measured by a spectrophotometer at 405 nm. The degree of color resulting from the reaction is proportional to the amount of Endotoxin in the test sample and can be calculated using a standard curve.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.