Alzheimer's Disease International estimates that dementia affects about 57 million people worldwide as of 2019, which is expected to nearly triple by 2050.1 Alzheimer's disease (AD) is characterized by the presence of β-amyloid peptide and phosphorylated tau protein (short for tubulin-associated unit2). Under normal conditions, tau is a microtubule-associated protein (MAP) involved in microtubule stabilization. Tau is found primarily in axons, where it regulates microtubule polymerization and stabilization.
Role of Tau Protein in Disease
In disease, tau becomes hyperphosphorylated and can no longer adequately stabilize the microtubules. Kinases such as GSK3β, CDK5, MAPK, CaMKII, and p38 carry out the phosphorylation step. Hyperphosphorylated tau forms insoluble filaments which accumulate as neurofibrillary tangles (NFTs). The resulting neurodegenerative diseases are called tauopathies and include progressive supranuclear palsy (PSP), Pick's disease (PiD), Parkinson's disease, and Alzheimer's disease.
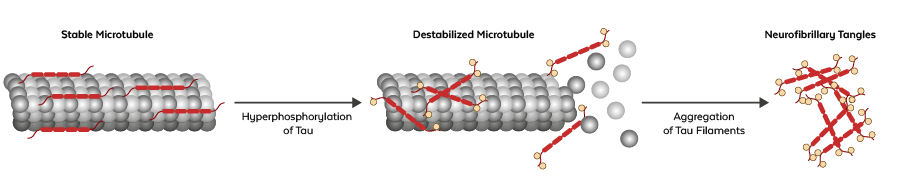
Figure: Formation of neurofibrillary tangles. Under pathological conditions, tau becomes hyperphosphorylated and destabilizes microtubules. Phosphorylated tau aggregates, builds filaments, and forms NFTs.
Advances in Tau Immunotherapy
Several tau antibodies and tau vaccines are currently in clinical trials or late-stage preclinical development. A large proportion of these are targeted for the treatment of AD, whereas most of these anti-tau antibodies are whole antibodies that work both intracellularly and extracellularly, blocking the spread of tau pathology via different mechanisms (see Sandusky-Beltran & Sigurdsson, 2020 for a review on past and present clinical trials). Recently, the results of another phase 1b study using a tau-targeting antisense oligonucleotide (MAPTRX) to reduce tau in patients with mild AD were published in Nature Medicine.5 While this is good news in itself, what is particularly noteworthy is that this is the first antisense oligonucleotide (ASO) treatment evaluated in a clinical study of patients with Alzheimer's. In this context, Rockland's recently introduced ModDetect™ Antibody Panels that allow for the detection of modified nucleic acids could further advance this innovative technology.
As we continue to identify interaction partners and modulators of tau, new opportunities will emerge to further improve efficacy. For example, another recent publication in Science showed that tau immunotherapy relies mainly on the intracellular antibody receptor TRIM21, which contributes to the neutralization of tau-antibody complexes.6 Despite the rapid development in this field, tau immunotherapies are of exceptional complexity. Rockland supports research in the fields of tauopathies and tau immunotherapy with specific antibodies and a wide range of full-length, mutated, and truncated tau proteins.
TAU Pathway Antibodies
| Product | Clonality | Reactivity | Application |
| CaM Kinase II Antibody | Polyclonal | Mouse | WB, ELISA |
| CaM Kinase II phospho T286 Antibody | Polyclonal | Mouse, Rat | WB |
| CaMKII Antibody | Monoclonal | Mouse, Rat, Bovine | WB, IHC, IF, IP |
| CaMKII Antibody | Monoclonal | Rat | WB, IHC, IF, IP |
| Cdk5 Antibody | Monclonal | Human, Mouse, Rat | WB, IHC |
| CDK5 Antibody | Polyclonal | Human, Mouse, Rat | WB, IHC |
| ERK-MAPK Antibody | Polyclonal | Human, Mouse, Rat | WB |
| ERK-MAPK phospho T202/phospho Y204 Antibody | Polyclonal | Human, Mouse, Rat | WB, IHC |
| GSK3 Beta phospho S9 Antibody | Polyclonal | Human | WB, IHC, ELISA |
| MAPK 8/9 Antibody | Polyclonal | Human, Mouse, Rat | WB, IHC |
| p38 Antibody | Polyclonal | Broad | WB, IHC, IF, IP |
| P38 Antibody | Polyclonal | Human, Mouse | WB, IHC, ELISA |
| p38 alpha MAPKinase Antibody | Monoclonal | Human | WB, IHC, IP |
| p38 MAPK phospho T180/phospho Y182 Antibody | Polyclonal | Human | WB, IHC |
| p70 S6 Kinase Antibody | Polyclonal | Mouse | WB, IP, ELISA |
| TAU Antibody | Polyclonal | Human, Rat | WB, IHC |
| Tau phospho S416 Antibody | Polyclonal | Mouse, Rat | WB, IHC |
| TRIM21 Antibody | Polyclonal | Human | WB, IHC, IF, ELISA |