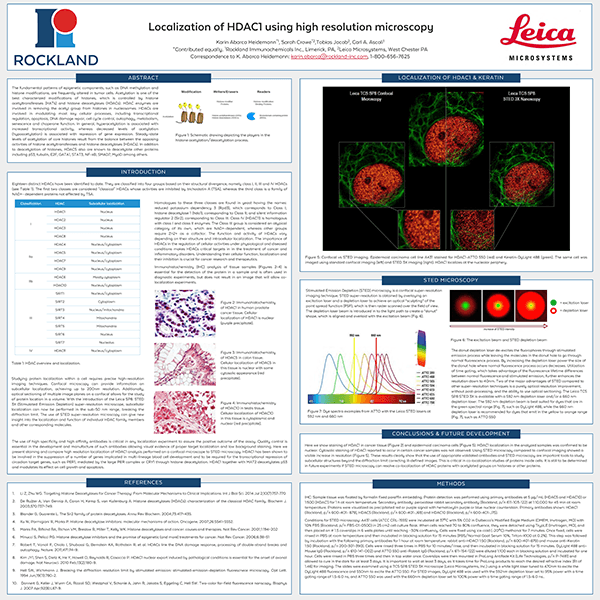
Localization of HDAC1 using high resolution microscopy
The fundamental patterns of epigenetic components, such as DNA methylation and histone modifications, are frequently altered in tumor cells. Acetylation is one of the best characterized modifications of histones, which is controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDAC enzymes are involved in removing the acetyl group from histones in nucleosomes. HDACs are involved in modulating most key cellular processes, including transcriptional regulation, apoptosis, DNA damage repair, cell cycle control, autophagy, metabolism, senescence and chaperone function.
In general, hyperacetylation is associated with increased transcriptional activity, whereas decreased levels of acetylation (hypoacetylation) is associated with repression of gene expression. Steady-state levels of acetylation of core histones result from the balance between the opposing activities of histone acetyltransferases and histone deacetylases (HDACs). In addition to deacetylation of histones, HDACS also are known to deacetylate other proteins including p53, tubulin, E2F, GATA1, STAT3, NF-kB, SMAD7, MyoD among others.