Human IgM ELISA Kit
KAA9107
1 Kit
ELISA
Human
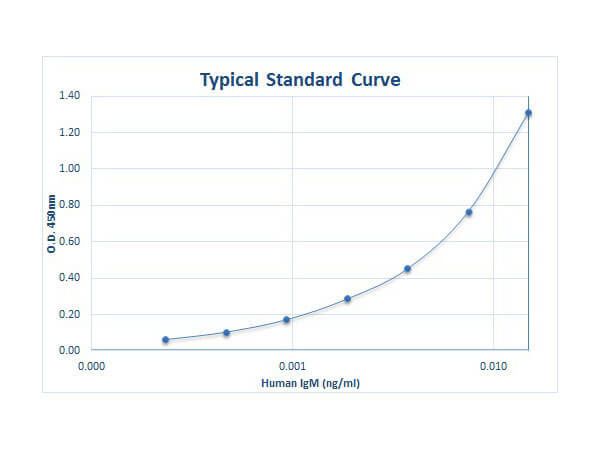
0.23 ng/ml - 15.0 ng/ml
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Human IgM AccuSignal ELISA Kit - KAA9107
Human IgM ELISA Kit
IgM (H&L)
Polyclonal
ELISA Kit
0.23 ng/ml - 15.0 ng/ml
Target Details
Human
Rockland’s Human IgM AccuSignal™ ELISA Kit is an in vitro enzyme-linked immunosorbent assay designed for the quantitative detection of human IgM in serum, plasma, and cell culture supernatants. This kit contains: Human IgM control, Capture antibody, biotinylated Detection Antibody, streptavidin-peroxidase conjugate, along with buffers and protocol. See protocol for details.
Application Details
ELISA
Human IgM ELISA kit is designed for the quantitative detection of human IgM in serum, plasma, and hybridoma cell supernatants.
Shipping & Handling
Wet Ice
See kit insert for complete instructions. Store coating buffer, wash buffer, blocking buffer, standard, and reagent diluent at 4˚C. Store substrate solution at 4°C prior to opening. Protect from moisture and light. Store detection antibody and SA-HRP conjugate vial at 4° C prior to restoration. Aliquot capture antibody and target protein standard and freeze at -20˚C. For extended storage aliquot contents and freeze at -20° C or below. Avoid multiple freeze-thaw cycles.
See kit insert for complete instructions.
IgM (immunoglobulin M) is the is the third most abundant and largest antibody in the human circulatory system. IgM is expressed on the surface of immature and mature B cells as a monomer and is expressed in a secreted form as a pentamer with very high avidity. IgM is the first human immunoglobulin to appear in response to initial exposure to an antigen and eliminates pathogens in the early stages of B cell-mediated (humoral) immunity before there is sufficient IgG. IgM is a strong complement activator and agglutinator due to its pentameric structure and binds fragment crystallization (Fc) receptors. IgM has a molecular mass of approximately 970 kDa in its pentamer form with ten binding sites. Typically, however, IgM cannot bind 10 antigens at the same time because the large size of most antigens hampers binding to neighboring sites. IgM antibodies do not pass across the human placenta. IgM antibodies are very useful in the diagnosis of infectious diseases. The presence of IgM antibodies in a patient’s serum indicates recent infection, or in a neonate’s serum specifies an intrauterine infection (e.g. congenital rubella).
No test method can provide total assurance that the hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or any other infectious agents are absent. Thus, all blood products, including purified proteins derived from human blood sources, should be handled at Biosafety Level 2 as recommended by the CDC\NIH manual entitled Biosafety in Microbiological and Biomedical Laboratories for potentially infectious human serum, blood specimens or proteins derived from same. Source material for the human blood product supplied to your facility has been tested for the detection of HIV antibody, Hepatitis B surface antigen, antibody to Hepatitis C, HIV 1 antigen(s), antibody to HTLV - I/II, and syphilis by FDA guidelines. All units were found to be non-reactive/negative for these tests. All human blood source material is collected in FDA licensed centers and is tested with FDA approved test kits.; This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.