Human IgG ELISA Kit
KAA9102
1 Kit
ELISA
Human
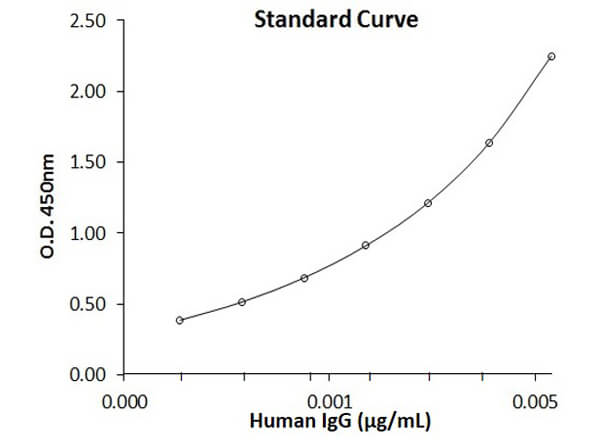
0.094 ng/ml - 6.0 ng/ml
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Human IgG AccuSignal ELISA Kit - KAA9102
Human IgG ELISA Kit
IgG (H&L)
Polyclonal
ELISA Kit
0.094 ng/ml - 6.0 ng/ml
Target Details
Human
Rockland’s human IgG AccuSignal™ ELISA Kit is an in vitro enzyme-linked immunosorbent assay designed for the quantitative detection of human IgG in serum, plasma, and hybridoma cell supernatants. This kit contains: Human IgG control, Capture antibody, biotinylated Detection Antibody, streptavidin-peroxidase conjugate, along with buffers and protocol. See protocol for details.
Application Details
ELISA
Human IgG ELISA kit is designed for the quantitative detection of human IgG in serum, plasma, and hybridoma cell supernatants.
Shipping & Handling
Wet Ice
See kit insert for component storage conditions. Store coating buffer, wash buffer, blocking buffer, standard, and reagent diluent at 4˚C. Store substrate solution at 4°C prior to opening. Protect from moisture and light. Store detection antibody and SA-HRP conjugate vial at 4° C prior to restoration. For extended storage aliquot contents and freeze at -20° C or below. Aliquot capture antibody and target protein standard contents and freeze at -20˚C. Avoid multiple freeze-thaw cycles.
See kit insert for complete instructions.
Immunoglobulin G (IgG) is the most abundant protein in the serum which accounts for 75% of the total immunoglobulins in the plasma of healthy individuals. IgG is a major effector molecule of the humoral immune response in humans and provides defense against parasitic invasion. IgG molecules (Fc portion) react with Fc-gamma receptors that are present on the surfaces of macrophages, neutrophils, natural killer cells, and can activate the complement system. IgG is produced in a delayed response to an infection and can be retained in the body for a long time. IgG is the major class of the five classes of immunoglobulins (IgM, IgD, IgG, IgA, and IgE) in human beings. These closely related glycoproteins, composed of 82–96% protein and 4–18% carbohydrate, differ in heavy chain structure and have unique profile with respect to antigen binding, immune complex formation, complement activation, triggering of effector cells and placental transport. IgG can be further divided into four subclasses, IgG1, IgG2, IgG3, and IgG4, based on their relative prevalence in human serum. The IgG concentration in normal human serum ranges from 6 mg/ml to 18 mg/ml. The relative abundance of the different immunoglobulin classes is generally constant in mature adults. Changes in the relative abundance of the different classes typically specify immunological disorders or disease states. For example, congenital and acquired immunodeficiency leads to significantly reduced levels of IgG, while hepatitis, cirrhosis, and most autoimmune diseases increase the levels of IgG. Thus, an accurate quantitation of IgG in plasma or serum is essential for diagnostic studies, as well as for monitoring the efficacy of treatment regimens.
No test method can provide total assurance that the hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or any other infectious agents are absent. Thus, all blood products, including purified proteins derived from human blood sources, should be handled at Biosafety Level 2 as recommended by the CDC\NIH manual entitled Biosafety in Microbiological and Biomedical Laboratories for potentially infectious human serum, blood specimens or proteins derived from same. Source material for the human blood product supplied to your facility has been tested for the detection of HIV antibody, Hepatitis B surface antigen, antibody to Hepatitis C, HIV 1 antigen(s), antibody to HTLV - I/II, and syphilis by FDA guidelines. All units were found to be non-reactive/negative for these tests. All human blood source material is collected in FDA licensed centers and is tested with FDA approved test kits.; This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.