Human Azurocidin ELISA Kit
KOA0667
1 Kit
ELISA
Human
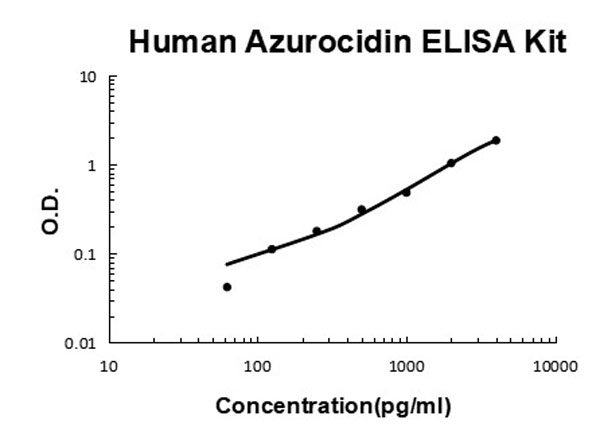
62.5 pg/ml - 4000 pg/ml
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Human Azurocidin AccuSignal ELISA Kit - KOA0667
Azu 1, AZU1, Azurocidin, CAP37, CAP7_HUMAN, Cationic antimicrobial protein 37, Cationic antimicrobial protein CAP37, HBP, Heparin binding protein, Heparin-binding protein, HUMAZUR, Neutrophil azurocidin
ELISA Kit
62.5 pg/ml - 4000 pg/ml
Target Details
AZU1 - View All AZU1 Products
Human
Expression system for standard: NSO; Immunogen sequence: I27-P248
Natural and recombinant human Azurocidin. There is no detectable cross-reactivity with other relevant proteins.
P20160 - UniProtKB
NP_001691.1 - NCBI Protein
Application Details
ELISA
Useful in Sandwich ELISA for Quantitative Detection of Antigen. Aliquot 0.1ml per well of the 4000pg/ml, 2000pg/ml, 1000pg/ml, 500pg/ml, 250pg/ml, 125pg/ml, 62.5pg/ml human Azurocidin standard solutions into the precoated 96-well plate. Add 0.1ml of the sample diluent buffer into the control well (Zero well). Add 0.1ml of each properly diluted sample of human cell culture supernates, serum or plasma(heparin, EDTA) to each empty well. It is recommended that each human Azurocidin standard solution and each sample be measured in duplicate.
Formulation
Heparin Sodium
Shipping & Handling
Wet Ice
Store vials at 4°C prior to opening. Centrifuge product if not completely clear after standing at room temperature. This product is stable for 6 months at 4°C as an undiluted liquid. Dilute only prior to immediate use. For extended storage freeze at -20°C or below for 12 months. Avoid cycles of freezing and thawing.
See kit insert for complete instructions.
Azurocidin, also known as cationic antimicrobial protein CAP37 or heparin-binding protein (HBP), is a protein that in humans is encoded by the AZU1 gene. This encoded protein is a member of the serine protease gene family, but it is not a serine proteinase, because the active site serine and histidine residues are replaced. Azurocidin is mapped to 19p13.3. The protein encoded by this gene is an azurophil granule antibiotic protein, with antibacterial activity. It is also an important multifunctional inflammatory mediator. In addition to it, Azurocidin is also a specific chemoattractant for monocytes. It lacks the chemotactic activity for neutrophils and lymphocytes, and this gene is probably responsible for the wave of monocytes that follows the initial wave of PMNs typical of the inflammatory response.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.