Guinea Pig Serum Sterile
34 References
D105-00-0050
D105-00-0100
D105-00-0500
D105-00-1000
50 mL
100 mL
500 mL
1 L
Liquid (sterile filtered)
Liquid (sterile filtered)
Liquid (sterile filtered)
Liquid (sterile filtered)
WB, ELISA, IHC, FC, Multiplex, Other
Guinea Pig
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Guinea Pig Serum (Sterile) - D105-00-0050
Guinea Pig serum for cell culture, cell culture grade guinea pig serum, sterile serum from guinea pig.
Guinea Pig
Application Details
ELISA, FC, IHC, Other, WB, Multiplex
- View References
pH: normal
Immunoelectrophoresis: normal
Hemoglobin: normal
IgG Concentration: normal
Tissue Data
Serum
Mixed
Guinea Pig - Mixed
Formulation
Sterile
72mg/ml by Refractometry
None
None
None
Shipping & Handling
Dry Ice
Store container at -20° C prior to opening. Avoid cycles of freezing and thawing. Use aseptic technique to maintain sterility when opening product.
Expiration date is one (1) year from date of receipt.
Guinea Pig Serum is used as a supplement to cell culture media. Guinea Pig Serum is also suitable for use as a component of bioassays, immunoassays or enzyme assays. Guinea Pig Serum provides a broad spectrum of macromolecules, carrier proteins for lipoid substances and trace elements, attachment and spreading factors, low molecular weight nutrients, and hormones and growth factors that promote cell growth and health. Be certain to maintain Good Cell Culture Practice, and maintain sterility of cultures that require media supplementation. Guinea Pig Serum is ideal for investigators in Cancer, Immunology, and Cell Biology research.
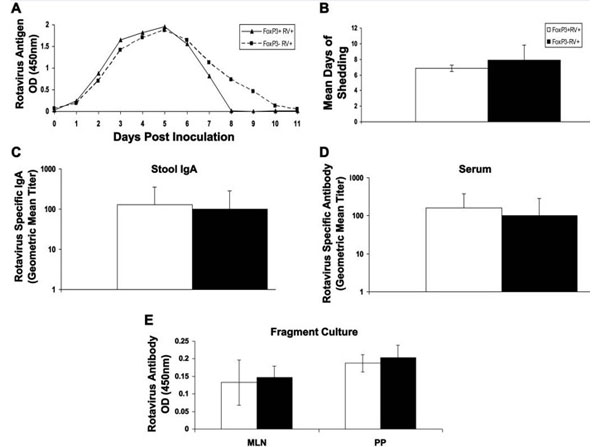
Miller AD et al. (2014). FoxP3+ regulatory T cells are not important for rotavirus clearance or the early antibody response to rotavirus. Microbes Infect.
Applications
E, EIA
Kanungo S et al. (2014). Safety and immunogenicity of a live oral recombinant cholera vaccine VA1.4: a randomized, placebo controlled trial in healthy adults in a cholera endemic area in Kolkata, India. PLoS One.
Applications
Other
Eisenstein TK et al. (2007). Anandamide and Δ9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J Neuroimmunol.
Applications
Other
Yotsushima K et al. (2004). Effects of fatty acid-free bovine serum albumin and fetal calf serum supplementing repair cultures on pre- and post-warm viability of biopsied bovine embryos produced in vitro. J Reprod Dev.
Applications
Other
Hsieh MH et al. (2000). T-cell subsets mediate graft-versus-myeloid leukemia responses via different cytotoxic mechanisms. Biol Blood Marrow Transplant.
Applications
FC, FACS, FLOW
Toma H et al. (1999). Immunohistochemical distribution of c-Kit-positive cells and nitric oxide synthase-positive nerves in the guinea-pig small intestine. J Auton Nerv Syst.
Applications
IHC, ICC, Histology; Multiplex Assay
Sawant-Mane S et al. (1996). CD59 homologue regulates complement-dependent cytolysis of rat Schwann cells. J Neuroimmunol.
Applications
Undefined
Edens RE et al. (1994). Heparin and derivatized heparin inhibit zymosan and cobra venom factor activation of complement in serum. Immunopharmacology.
Applications
Other
Sawant-Mane S et al. (1994). Antibody of patients with Guillain-Barré syndrome mediates complement-dependent cytolysis of rat Schwann cells: susceptibility to cytolysis reflects Schwann cell phenotype. J Neuroimmunol.
Applications
Undefined
Beck LA et al. (1988). Incorporation of a product of mevalonic acid metabolism into proteins of Chinese hamster ovary cell nuclei. J Cell Biol.
Applications
WB, IB, PCA
Lin YS et al. (1986). Inhibition of antibody production from the plasmacytoma cell line MOPC-315 by staphylococcal enterotoxin B-induced T-suppressor cells. Cell Immunol.
Applications
Undefined
Whitlow MB et al. (1985). Penetration of C8 and C9 in the C5b-9 complex across the erythrocyte membrane into the cytoplasmic space. J Biol Chem.
Applications
Other
Ishii Y et al. (1985). Translation of human macrophage activating factor (for glucose consumption) mRNA in Xenopus laevis oocytes. Immunol Invest.
Applications
Undefined
Ramm LE et al. (1983). Size distribution and stability of the trans-membrane channels formed by complement complex C5b-9. Mol Immunol.
Applications
Undefined
Ramm LE et al. (1983). Elimination of complement channels from the plasma membranes of U937, a nucleated mammalian cell line: temperature dependence of the elimination rate. J Immunol.
Applications
Undefined
Rosenfeld SI et al. (1983). Inhibition of the lytic action of cell-bound terminal complement components by human high density lipoproteins and apoproteins. J Clin Invest.
Applications
Other
Imagawa DK et al. (1983). Consequences of cell membrane attack by complement: release of arachidonate and formation of inflammatory derivatives. Proc Natl Sci USA
Applications
Other
Jordan RE et al. (1983). Antithrombin in vertebrate species: conservation of the heparin-dependent anticoagulant mechanism. Arch Biochem Biophys.
Applications
Undefined
Ramm LE et al. (1982). Size of the transmembrane channels produced by complement proteins C5b-8. J Immunol.
Applications
Undefined
Ramm LE et al. (1982). Transmembrane channel formation by complement: functional analysis of the number of C5b6, C7, C8, and C9 molecules required for a single channel. Proc Natl Sci USA
Applications
Other
Campos-Neto A. (1982). Immune response gene control of antibody specificity. Cell Immunol.
Applications
Undefined
Bucy RP et al. (1981). Ir gene control of the immune response to insulins. I. Pork insulin stimulates T cell activity in nonresponder mice. J Immunol.
Applications
Undefined
Ramm LE et al. (1980). Life-span and size of the trans-membrane channel formed by large doses of complement. J Immunol.
Applications
Undefined
Cines DB et al. (1979). Effect of anti-P1A1 antibody on human platelets. I. The role of complement. Blood.
Applications
Other
Campos-Neto A. (1978). T-cell regulation of restricted B-cell responses. Adv Exp Med Biol.
Applications
Undefined
MacGregor RR et al. (1978). Impaired granulocyte adherence in multiple myeloma: relationship to complement system, granulocyte delivery, and infection. Blood.
Applications
Other
Schreiber AD et al. (1978). Effect of C3b inactivator on monocyte-bound C3-coated human erythrocytes. Blood.
Applications
Other
Baltz M et al. (1977). Low-dose antigenic signals to helper lymphocytes. Cell Immunol.
Applications
Cell Depletion
Steshenko PP et al. (1977). Antigen recognition: the specificity of an isolated T lymphocyte population. J Immunol.
Applications
Undefined
Yaron A et al. (1977). Immune Ascites in the Guinea Pig: Specificity of Cells and Antibody in an Induced Peritoneal Exudate. J Immunol.
Applications
Undefined
Baltz M et al. (1977). Down regulation in B lymphocytes: low dose signals. Eur J Cell Biol.
Applications
Cell Depletion
Schreiber AD et al. (1976). Acquired angioedema with lymphoproliferative disorder: association of C1 inhibitor deficiency with cellular abnormality. Blood.
Applications
IHC, ICC, Histology; Other
Kenna MA et al. (1975). Effect of papain on the interaction between human monocytes, erythrocytes, and IgG. Blood.
Applications
Other
Twomey SL et al. (1974). Shortened Radioimmunoassay for Human Growth Hormone. Clin Chem.
Applications
Undefined
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.