p53 Pathway Antibodies
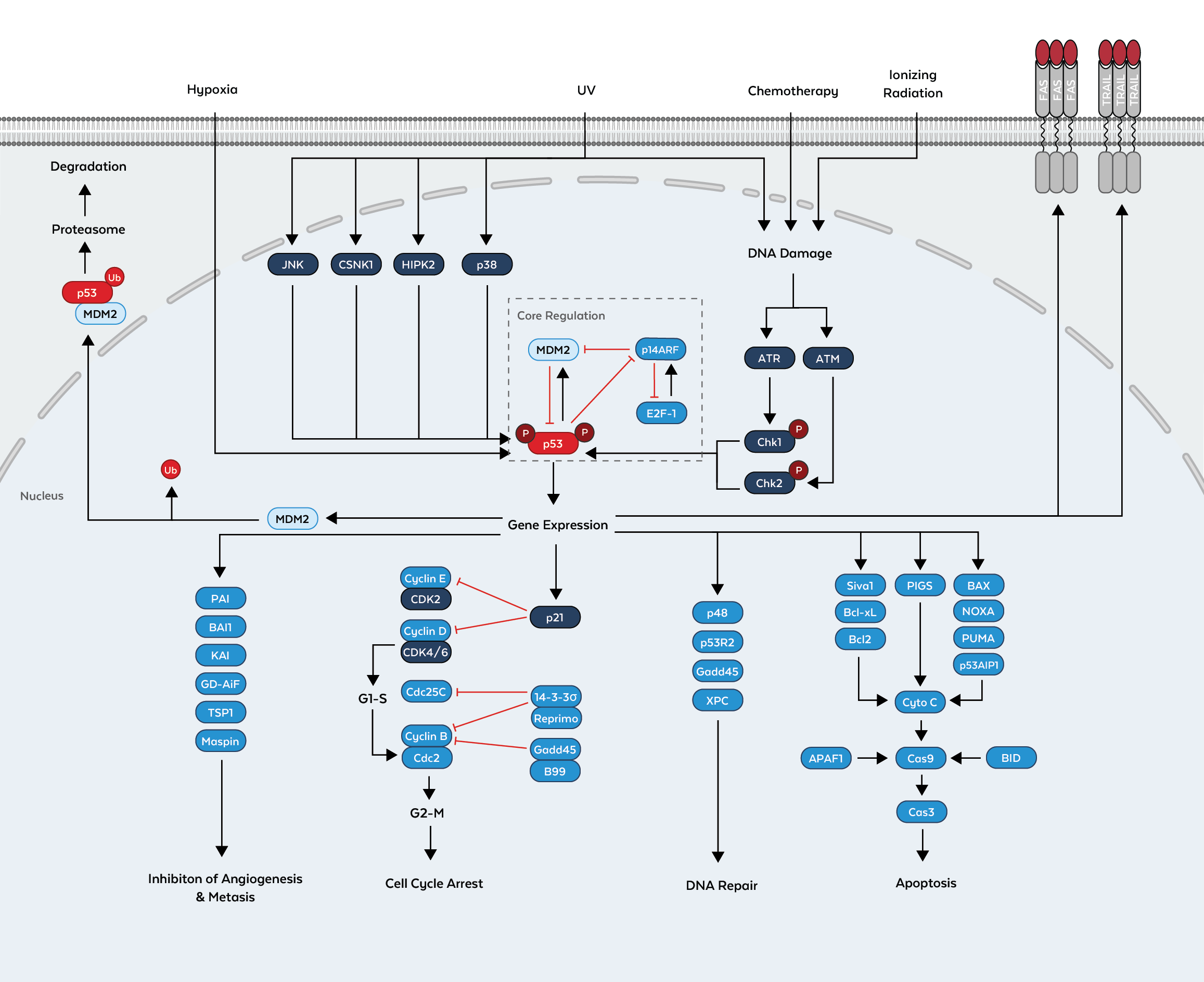
p53 is a tumor suppressor protein encoded in humans by the TP53 gene. It is a crucial component in multicellular organisms, as it regulates the cell cycle and helps prevent cancer. p53 is the most frequently altered gene in human cancers. The name is due to its molecular mass, which is in the 53 kilodalton fraction of cell proteins. p53 has many anti-cancer mechanisms of and plays a major role in the inhibition of angiogenesis. As an anti-cancer promotion agent, it can activate DNA repair proteins when DNA has sustained damage, induce growth arrest by holding the cell cycle at the G1/S regulation point on DNA damage recognition, or initiate apoptosis if DNA damage proves to be irreparable.
The mechanisms by which p53 leads the cell to cell growth arrest or apoptosis remain poorly understood. Evidence suggests that the in vivo functions of p53 seem to balance the cell-fate choice with the type and severity of damage that occurs. The antirepression or inhibition factors that normally keep p53 at balance provide novel chemotherapeutic targets for the reactivation of p53 in tumors harboring a wild-type copy of the gene. Further, studies of human embryonic stem cells (hESCs) commonly describe the nonfunctional p53-p21 axis of the G1/S checkpoint pathway with subsequent relevance for cell cycle regulation and the DNA damage response (DDR). P21 mRNA is clearly present and upregulated after the DDR in hESCs, but p21 protein is not detectable, as in this cell type, p53 activates numerous microRNAs that directly inhibit the p21 expression in hESCs.
Additionally, recent research has also linked the p53 and RB1 pathways, via p14ARF, raising the possibility that the pathways may regulate each other. By regulating LIF, p53 has been shown to facilitate implantation in the mouse model and possibly in humans. p53 expression can be stimulated by UV light, which also causes DNA damage.
p53 Signaling Pathway
p53 is maintained at low protein levels during times of homeostasis, when the cell is not exposed to stress or DNA-damaging events, by its predominant negative regulator Mdm2 through the ubiquitin-proteasome pathway. The Mdm2 E3 ubiquitin ligase represses p53 protein levels through continuous ubiquitination and degradation. The Mdm2-p53 interaction is inhibited by stress-induced acetylation and phosphorylation on Mdm2 by the kinases ATM and c-Abl. A number of phosphorylationand acetylation sites on p53 have been described and many serve to disrupt the Mdm2-p53 interaction as well. Once activated, p53 will induce a cell cycle arrest to allow either repair and survival of the cell or apoptosis. Activated p53 binds DNA and activates expression of several genes including WAF1/CIP1 encoding for p21. p21 (WAF1) binds to the G1-S/CDK (CDK2) and S/CDK complexes (molecules important for the G1/S transition in the cell cycle) inhibiting their activity. When p21(WAF1) forms a complex with CDK2 the cell cannot continue to the next stage of cell division. A mutant p53 will no longer bind DNA in an effective way, and, as a consequence, the p21 protein will not be available to act as the "stop signal" for cell division.
Uses of p53 Pathway Products
The P53 pathway is important in cancer research. If the TP53 gene is damaged, tumor suppression is severely reduced. People who inherit only one functional copy of the TP53 gene will most likely develop tumors in early adulthood. The TP53 gene can also be damaged in cells by mutagens, increasing the likelihood that the cell will begin decontrolled division. More than 50 percent of human tumors contain a mutation or deletion of the TP53 gene. Restoring endogenous p53 function holds a lot of promise, while loss of p53 creates genomic instability that most often results in the aneuploidy phenotype.
Certain pathogens can affect the p53 protein .One such example, human papillomavirus (HPV), encodes the E6 protein, which binds to the p53 protein and inactivates it, which in conjunction with the inactivation of a number of other regulating factors, leads to the clinical disease of warts. Certain HPV types, in particular types 16 and 18, can also lead to progression from a benign wart to low or high-grade cervical dysplasia, which are reversible forms of precancerous lesions.
In healthy humans, the p53 protein is continually produced and degraded in the cell. The degradation of the p53 protein is associated with MDM2 binding. In a negative feedback loop, MDM2 is itself induced by the p53 protein, however, mutant p53 proteins often do not induce MDM2, and are thus able to accumulate at very high concentrations.