H5N1 Evolution and the Importance of Hemagglutinin (HA)
Recent developments in the spread of the H5N1 avian influenza (commonly known as bird flu) virus have raised global concerns, marking a significant shift in the virus's epidemiology. Initially identified over two decades ago, H5N1, a strain of highly pathogenic avian influenza (HPAI), has undergone substantial genetic changes, enhancing its pathogenicity and expanding its host range. Notably, the virus has demonstrated the capability to infect a wide array of avian species and mammals, including humans, posing a potential risk of a future pandemic.1
As H5N1 avian influenza continues to circulate globally, researchers are working to understand and control emerging strains to reduce this risk. These new strains challenge the effectiveness of current preventative measures. In a recent publication, researchers unveiled how current intramuscular (IM) and RNA vaccines present limitations, while intranasal (IN) vaccines may offer a more promising avenue for a pandemic response.2
In light of these developments, research efforts have intensified to understand the molecular mechanisms of H5N1 pathogenicity and transmissibility. A critical area of focus is the hemagglutinin (HA) protein, a major surface antigen of the influenza virus that mediates entry into host cells.
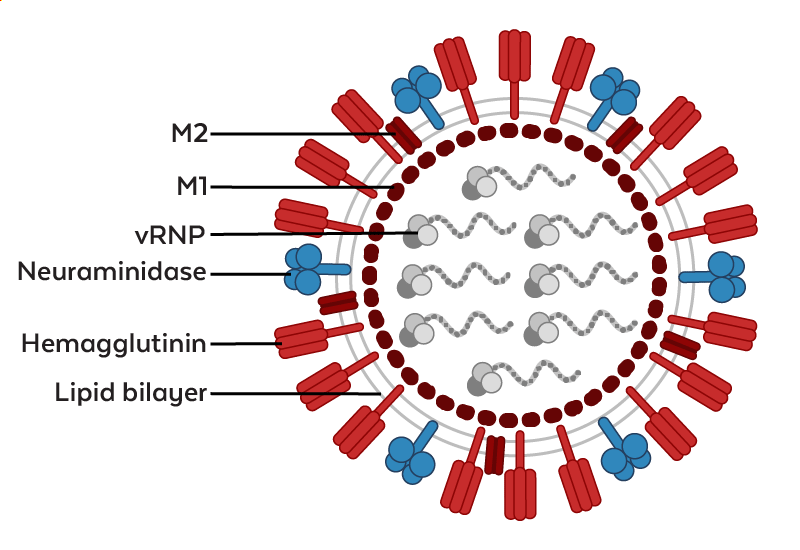
Figure. Schematic representation of an influenza A virus (Adapted from Eichberg et al., 2022)
Rockland H5N1 HA Antibodies Validated in Vaccine Research
Rockland's advanced suite of antibodies against the H5N1 HA protein offers a vital tool for scientists aiming to map the antigenic landscape of H5N1 and develop strategies for both therapeutic interventions and vaccines.
To validate these approaches, monovalent and bivalent intranasal vaccines expressing full-length H5 and H7 hemagglutinins (HA) from relevant influenza strains were analyzed using Rockland’s anti-HA A/Vietnam/1203/04 influenza virus (VN04-8) antibody as a positive control in H5-specific ELISAs.2 The results demonstrated a positive mucosal immune response with no competitive features between the two viruses, supporting the use of Rockland antibodies in influenza immunogenicity research.
While the experiment evaluated older strains, it was intended to establish intranasal RNA vaccine technology using existing reagents and animal efficacy models, with the knowledge that RNA vaccines allow for rapid reformulation against emerging antigen targets when required.3 Overall, the study highlights how intranasal RNA vaccines can provide a versatile platform capable of quickly adapting to newly emerging influenza strains, supported by reliable research reagents.
H5N1 Antibody Cross-Reactivities
| HA Clade | H5N1 Influenza Virus | HI titers with anti-HA monoclonal antibodies | |||||
| VN04-2 | VN04-8 | VN04-9 | VN04-10 | VN04-13 | VN04-16 | ||
| H5 Ref. | A/tern/South Africa/1961 | 100 | < | < | < | < | < |
| North American | A/chicken/Pennsylvania/1370/1983 | 3200 | < | 25600 | 200 | 3200 | < |
| A/mallard/Pennsylvania/10218/1984 | 800 | < | 200 | 6400 | 25600 | 400 | |
| A/chicken/Hidalgo/28159-232/1994 | < | < | 200 | 100 | 1600 | < | |
| A/mallard/Arkansas/1/2001 | 1600 | < | 200 | 400 | 3200 | 100 | |
| Clade 0 | A/Hong Kong/156/1997 | 6400 | < | 25600 | 6400 | 25600 | 400 |
| A/Hong Kong/481/1997 | 6400 | < | 1600 | 1600 | 12800 | 100 | |
| A/duck/Singapore/3/1997 | 200 | < | 200 | 800 | 6400 | 200 | |
| A/goose/Hong Kong/437-4/1999 | 6400 | < | 6400 | 1600 | 6400 | 200 | |
| Clade 1 | A/Vietnam/1194/2004 | 3200 | 1600 | 12800 | 3200 | 6400 | 1600 |
| A/Vietnam/1203/2004 | 6400 | 1600 | 12800 | 3200 | 6400 | 1600 | |
| A/Vietnam/HN30408/2005 | 6400 | 3200 | 3200 | 3200 | 6400 | 1600 | |
| A/Hong Kong/213/2003 | 6400 | 3200 | 400 | 3200 | 800 | 3200 | |
| Clade 2.1.2 | A/Indonesia/6/2005 | 3200 | < | 800 | 25600 | 200 | 6400 |
| Clade 2.1.3 | A/Indonesia/5/2005 | < | < | 400 | 12800 | 200 | 3200 |
| A/chicken/Indonesia/PA03/2003 | 800 | 3200 | 200 | 3200 | 1600 | 1600 | |
| A/duck/HUNWG/1504/2004 | 1600 | < | 3200 | 1600 | < | 400 | |
| A/duck/GXLA/1304/2004 | < | 1600 | < | 3200 | 1600 | 1600 | |
| A/chicken/Jogjakarta/BBVET/IX/2004 | 100 | < | 100 | 3200 | 3200 | 400 | |
| A/chicken/Malang/BBVET/IV/2004 | 3200 | 3200 | < | 3200 | 3200 | 1600 | |
| Clade 2.2 | A/whopper swan/Mongolia/244/2005 | < | 1600 | < | 3200 | 1600 | 1600 |
| A/turkey/15/2006 | 100 | < | < | 3200 | < | 400 | |
| A/bar headed goose/Qinghai/1A/2005 | 100 | 6400 | < | 6400 | 12800 | 3200 | |
| Clade 2.3.4 | A/duck/Hunan/15/2004 | 1600 | < | 3200 | 1600 | < | 400 |
| A/duck/Laos/3295/2006 | < | < | 400 | 1600 | 100 | 100 | |
| A/chicken/Malaysia/935/2006 | 100 | < | 400 | 800 | 100 | 100 | |
| A/common magpie/Hong Kong/645/2006 | < | < | 200 | 400 | < | 100 | |
| Clade 2.4 | A/duck/Guangxi/13/2004 | < | 1600 | < | 3200 | 1600 | 1600 |
Table 1: Hemagglutination inhibition (HI) testing was performed with 0.5% chicken red blood cells by a standard method.4 (<) less than 1:100.
H5N1 Antibodies
| Product | Clonality | Clone | Size |
| H5N1 Antibody VN04-2 | Monoclonal | 15A3 | 100 µL |
| H5N1 Antibody VN04-2 | Monoclonal | 15A3 | 100 µg |
| H5N1 Antibody VN04-8 | Monoclonal | 3G2 | 100 µL |
| H5N1 Antibody VN04-8 | Monoclonal | 3G2 | 100 µg |
| H5N1 Antibody VN04-9 | Monoclonal | 7A11 | 100 µL |
| H5N1 Antibody VN04-9 | Monoclonal | 7A11 | 100 µg |
| H5N1 Antibody VN04-10 | Monoclonal | 8A3 | 100 µL |
| H5N1 Antibody VN04-10 | Monoclonal | 8A3 | 100 µg |
| H5N1 Antibody VN04-13 | Monoclonal | 14C5 | 100 µL |
| H5N1 Antibody VN04-13 | Monoclonal | 14C5 | 100 µg |
| H5N1 Antibody VN04-16 | Monoclonal | 18E1 | 100 µL |
| H5N1 Antibody VN04-16 | Monoclonal | 18E1 | 100 µg |
| H5N1 Influenza Antibody Sampler Kit | Monoclonal | Various | 1 kit |