H5N1 Antibody VN04-13
Mouse Monoclonal 14C5 IgG2a kappa
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Background
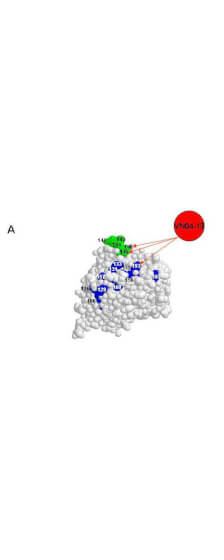
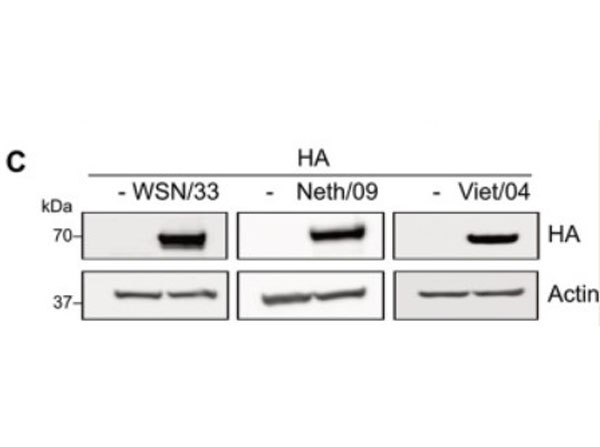
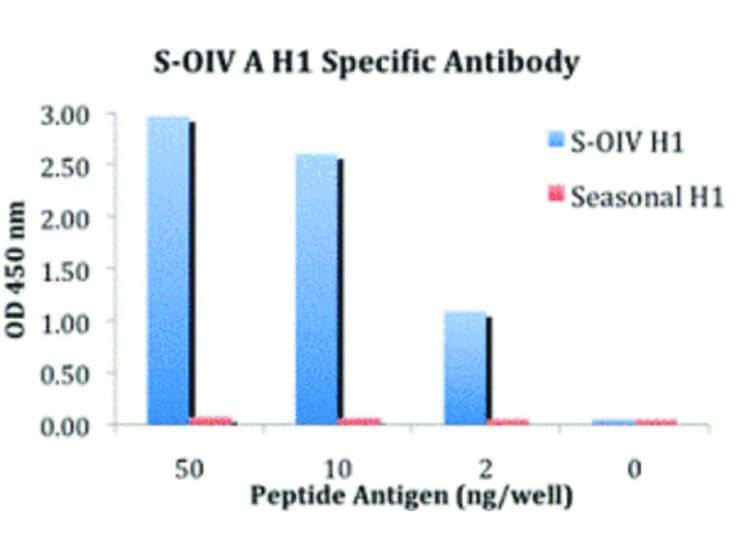
Hemagglutinin of A/Vietnam/1203/04 Influenza Virus (VN04-13) Antibody raised against the hemagglutinin (HA) surface glycoprotein of the A/Vietnam/1203/04 (H5N1) influenza virus. Generally referred to as ''bird flu'', the H5N1 influenza A virus has been documented in poultry and humans across ten Eurasian countries, from Japan in the north to Indonesia in the south. Without immunity, humans would have no protection against H5N1 influenza viruses, which could potentially cause a catastrophic pandemic influenza. This antibody, directed against the HA surface glycoprotein of the A/Vietnam/1203/04 (H5N1) influenza virus, is intended to further our understanding of the mechanisms underlying antigenic variation and evolution of novel variants. The major functions of HA include receptor-binding and fusion activities, but there may also be a structural role for HA in viral particle formation. Following attachment of HA to surface receptors on susceptible cells, the influenza virus enters the cell via endocytosis and membrane fusion.
Product Details
Target Details
Application Details
Formulation
Shipping & Handling
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.