Human Collagen Type II
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Target Details
Application Details
Formulation
Shipping & Handling
What is the source of the collagen proteins?
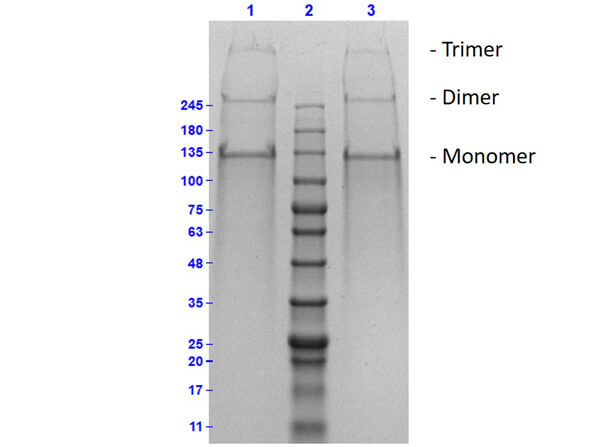
Collagen proteins are purified from various sources including bovine or human placenta, skin, nasal tissue, and knee cartilage. The purity of the protein is analyzed by SDS-PAGE.
How are the collagen proteins purified?
The proteins were purified by a proprietary method that includes mechanical separation and salt precipitation. Due to the nature and close association of certain collagen proteins, it is difficult to fully separate each collagen.
In what format are the collagen proteins provided?
Collagen proteins are provided in liquid format at a concentration of around 1 mg/mL. The collagen proteins are solubilized, maintaining their native structure. No enzymatic treatment is performed. The low pH of the buffer is essential in maintaining the solubility of the collagen protein.
In what buffer are the collagen proteins provided?
0.5 M Acetic Acid, pH 2.5 with 0.01% (w/v) Sodium Azide.
What are the recommended SDS-PAGE conditions for collagen proteins?
The recommended buffer for SDS-PAGE is 2X SDS buffer with 8 M Urea. The low pH of the buffer maintains the solubility of the collagen proteins. Also, low-density gels such as 3-8% Tris Acetate should be used. Collagen proteins can be reduced with DTT.
What are the recommended storage conditions for collagen proteins?
Collagen proteins should always be stored at 4°C. It is important to never let the collagen proteins freeze because this will degrade the protein. Please be sure to maintain 4°C temperature during assays such as SDS-PAGE.
How was the concentration determined?
The concentration of the collagen proteins was measured by absorbance via nanodrop at 205 nm. When measuring concentration, please be sure to blank with the same buffer as the protein product (0.5 M Acetic Acid).
Are collagen proteins suitable for cell culture?
Since no testing for endotoxin or other contaminants was performed, we do not recommend the use of this product for cell culture.