Datasheet is currently unavailable. Try again or CONTACT US
HSP90 Antibody
Mouse Monoclonal AC-16 IgG2b
200-301-F67
200 µg
Liquid (sterile filtered)
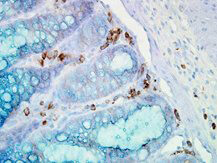
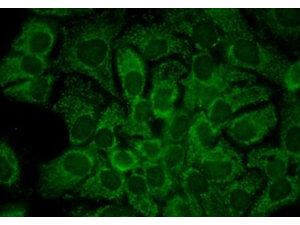
WB, IHC
Human, Mouse, Rat, Achyla, Chicken, Plants, Rabbit, Sf9, Wheat Germ
Mouse
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Anti-HSP90 (MOUSE) Monoclonal Antibody - 200-301-F67
Hsp84, Hsp90, Hsp90 beta, Hsp90B, HspC2, HSPCB, Heat shock protein HSP 90-beta, HSP 90, Heat shock 84 kDa, HSP 84, HSP84, HSP90AB1, HSP90B, HSPC2, HSPCB
Mouse
Monoclonal
IgG2b
Target Details
HSP90AA1 - View All HSP90AA1 Products
Human, Mouse, Rat, Achyla, Chicken, Plants, Rabbit, Sf9, Wheat Germ
Other
Hsp90 Antibody was produced in mice by repeated immunizations raised against heat shock protein 90 from the water mold Achyla ambisexualis.
Anti-Hsp90 Antibody was purified by Protein G chromatography. A BLAST analysis was used to suggest cross-reactivity with Hsp90 from Human, Rabbit, Rat, Mouse, Chicken, Achyla, Wheat Germ, and Sf9 cell line based on 100% homology with the immunizing sequence. Cross-reactivity with Hsp90 from other sources has not been determined. Heat Shock research.
Application Details
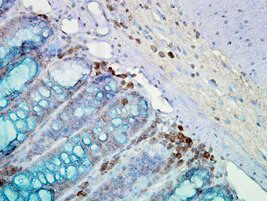
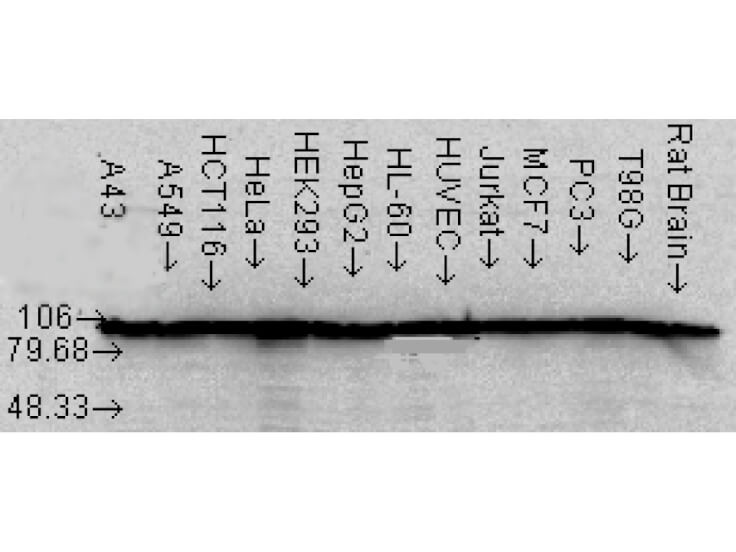
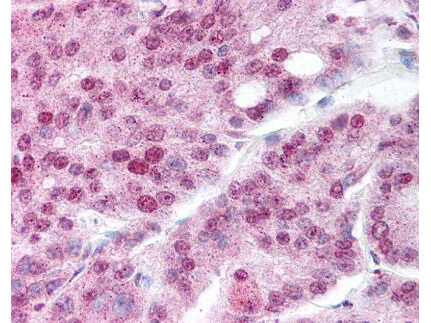
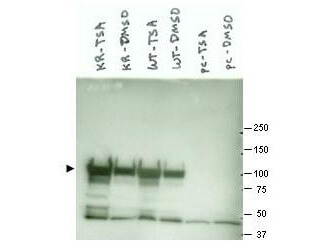
IHC, WB
Anti-Hsp90 Antibody is tested for WB and IHC. This antibody is reactive with both the constitutive and the inducible form of Hsp90. It does not bind to the native form and does not recognize Hsp90 from E.coli or yeast. Specific conditions for reactivity should be optimized by the end user.
Formulation
1mg/mL by UV absorbance at 280 nm
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.09% (w/v) Sodium Azide
50% (v/v) Glycerol
Shipping & Handling
Dry Ice
Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use.
Expiration date is one (1) year from date of receipt.
Hsp90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. From a functional perspective, hsp90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex. Despite its label of being a heat-shock protein, hsp90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the hsp90- regulated proteins that have been discovered to date are involved in cell signaling. The number of proteins now know to interact with Hsp90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5 When bound to ATP, Hsp90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, hsp90-interacting proteins have been shown to co-precipitate with hsp90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in hsp90 expression or hsp90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit hsp90 function.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.