Wood Breast Cancer Cell Model
Background
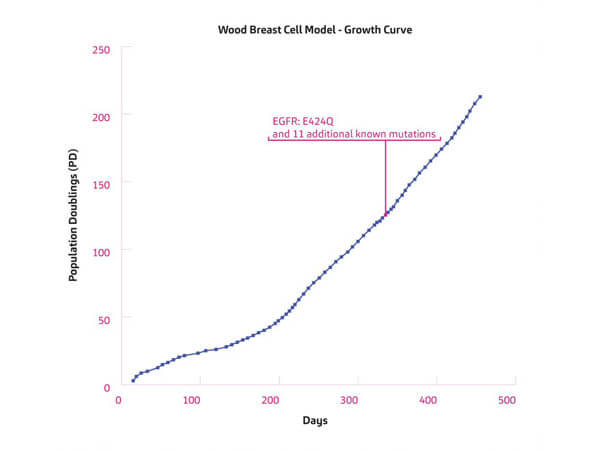
Wood Breast Cell Model originates from an infiltrating ductal and lobular carcinoma of the breast of a 65-69 yr old caucasian female collected in the United States in 2013. STR Profile; AMEL: X; CSF1PO: 11, 13; D13S317: 9, 14; D16S539: 12, 13; D18S51: 13, 15; D21S11: 29, 31; D3S1358: 14, 18; D5S818: 11, 12; D7S820: 10, 11; D8S1179: 12, 13; FGA: 24, 26; Penta D: 11, 13; Penta E: 13, 14; TH01: 7, 9; TPOX: 9, 11; vWA: 18. Manufactured by Cellaria, LLC.
Product Details
Target Details
Application Details
Cell Line Data
Formulation
Shipping & Handling
No test method can provide total assurance that the hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or any other infectious agents are absent. Thus, all blood products, including purified proteins derived from human blood sources, should be handled at Biosafety Level 2 as recommended by the CDC\NIH manual entitled Biosafety in Microbiological and Biomedical Laboratories for potentially infectious human serum, blood specimens or proteins derived from same. Source material for the human blood product supplied to your facility has been tested for the detection of HIV antibody, Hepatitis B surface antigen, antibody to Hepatitis C, HIV 1 antigen(s), antibody to HTLV - I/II, and syphilis by FDA guidelines. All units were found to be non-reactive/negative for these tests. All human blood source material is collected in FDA licensed centers and is tested with FDA approved test kits.; This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.