Guinea Pig Complement (lyophilized) with DILUENT
4 References
C200-0005
5 mL
Lyophilized
ELISA, Other, Purification
Guinea Pig
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Guinea Pig Complement (Lyophilized) with DILUENT - C200-0005
Complement system, tissue macrophages, blood monocytes, protease C3-convertase, mannose-binding lectin pathway, C3, C3a, C3b, C5a, C5b, C6, C7, C8, and polymeric C9, cascade cleavage and activation events, recruit inflammatory cells, anaphylatoxin
Guinea Pig
Application Details
ELISA, Other, Purification
- View References
Guinea Pig Complement (Lyophilized) with Diluent is designed for use in a range of immunological applications, including complement fixation tests (CFT) and serum radial hemolysis (SRH). It exhibits standard physiological properties such as normal pH, immunoelectrophoresis, hemoglobin, and IgG concentration, ensuring reliability across experiments. Ideal for ELISA, neutralization, and other applications, the complement shows robust activity with low cytotoxic background.
Tissue Data
Complement
Mixed
Guinea Pig - Mixed
Formulation
Non-sterile
85mg/ml by Refractometry
None
None
None
5.0 mL
Restore with Diluent
Shipping & Handling
Ambient
Store Guinea Pig Complement at 4° C prior to restoration. Aliquot contents and freeze at -70° C or below. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. COMPLEMENT IS A TEMPERATURE SENSITIVE PRODUCT. IMPROPER STORAGE WILL INACTIVATE COMPLEMENT ACTIVITY.
Expiration date is one (1) year from date of receipt.
Guinea Pig Complement (Lyophilized) with Diluent from Rockland Immunochemicals is optimized for high complement activity, making it an essential component for immunological applications such as immunogenicity assays and vaccine studies. It is derived from guinea pig serum, offering a robust solution for various immunological applications. This product includes a diluent for reconstitution, ensuring ease of use and consistency across applications.
Albert JR et al. (2020). Maternal DNMT3A-dependent de novo methylation of the paternal genome inhibits gene expression in the early embryo. Nat Commun.
Applications
Other
Timiryasova TM, Luo P, Zheng L, et al. (2019). Rapid fluorescent focus inhibition test optimization and validation: Improved detection of neutralizing antibodies to rabies virus. J Immunol Methods.
Applications
E, EIA
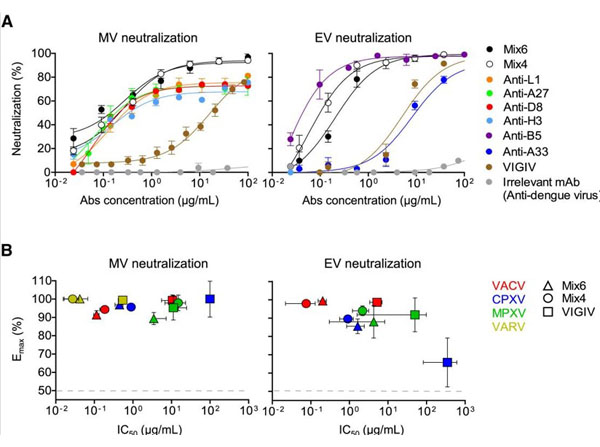
Gilchuk I, Gilchuk P, Sapparapu G, et al. (2016). Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell.
Applications
NEU
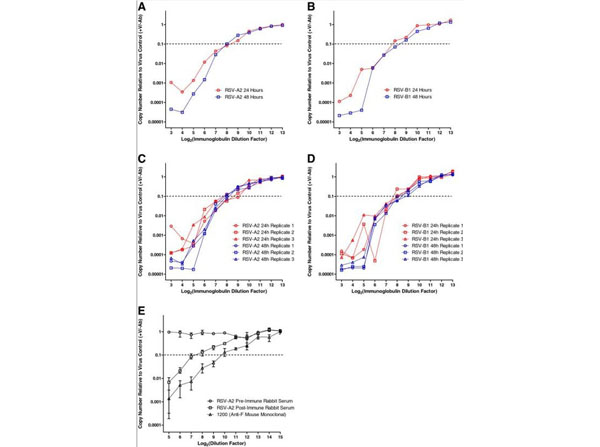
Varada JC, Teferedegne B, Crim RL, et al. (2013). A neutralization assay for respiratory syncytial virus using a quantitative PCR-based endpoint assessment. Virol J.
Applications
NEU
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.